Frontiers in Functional Genomics
Combatting SNAREopathies
Spearheaded by Professor Matthijs Verhage, neurodevelopmental disorders collectively referred to as ‘SNAREopathies’ have been a focus of research at Amsterdam UMC / Vrije Universiteit for decades. With the establishment of the European STXBP1 Consortium (ESCO), the section Functional Genomics will lead an international effort to bring hope and novel solutions for patients with these rare genetic disorders. In 2023, ESCO made significant steps forward by securing consortium agreement signatures of all academic and industry partners, obtaining its first funding, and filing an application at the European Medicines Agency (EMA) to facilitate patient research.
How do your brain cells (neurons) talk to each other?
Imagine little bubbles called ‘synaptic vesicles.’ These vesicles carry messages in the form of chemicals (neurotransmitters) that need to be delivered from one neuron to another across a tiny gap called the synaptic cleft. But for the message to be delivered, it first needs to get outside of the cell, across the cell membrane.
SNARE protein and their partners (the SNARE complex) play a crucial role in ensuring that the synaptic vesicles dock properly at the right spot on the neuron’s membrane and merge (fuse) with it, releasing the neurotransmitters into the synaptic cleft. Without this process, called ‘exocytosis,’ neurons cannot effectively communicate with each other. The neurotransmitters then bind to receptors on the neighboring neuron, delivering their message.
Mutations in genes coding for any of the SNARE-complex genes leads to decreased vesicle exocytosis. In humans, this gives rise to a spectrum of neurodevelopmental disorders, collectively referred to as ‘SNAREopathies’, marked by intellectual disability, movement disorders, and epilepsy.
STXBP1: A common culprit
The STXBP1 gene is currently one of the most commonly implicated genes in SNAREopathies. It encodes the ‘syntaxin-binding protein 1’, which helps ensure that neurotransmitter release is precisely timed and executed. This regulation is crucial for everything from basic sensory processing to complex cognitive functions.
Pathogenic variants (mutations) in STXBP1 were first discovered in 2008 in infants with severe epilepsy. Since then, disruptions to this gene have been linked with a broad array of symptoms, such as early-onset seizures, developmental delays, intellectual disabilities, and features of autism spectrum disorder. With an incidence rate of 1 in 30,000, STXBP1-related disorders (STXBP1-RD) are one of the most prevalent monogenic neurodevelopmental disorders.
Currently, there are no curative or specific therapies available for individuals with STXBP1-RD. Treatments are usually aimed at controlling the symptoms, such as anti-seizure medicines or ketogenic diets, although this does not help all patients.
Functional Genomics
Led by Professor Matthijs Verhage, STXBP1 has been a focus of research at Amsterdam UMC / Vrije Universiteit for decades. Prof. Verhage and his team have been investigating STXBP1 since 1993, publishing over 50 scientific reports on its function in neurotransmission.
In 2001, he founded the Functional Genomics Department at Vrije Universiteit, now a section of the Department of Human Genetics at Amsterdam UMC in addition to its affiliation with the Life Sciences faculty of Vrije Universiteit.
Prof. Verhage and his team’s contributions to STXBP1 research include pioneering mouse models, patient mutation analyses, and patient-derived neuron studies. Currently the group focuses on developing additional animal and human disease models and testing new therapies for STXBP1-RD.

The power of collective action
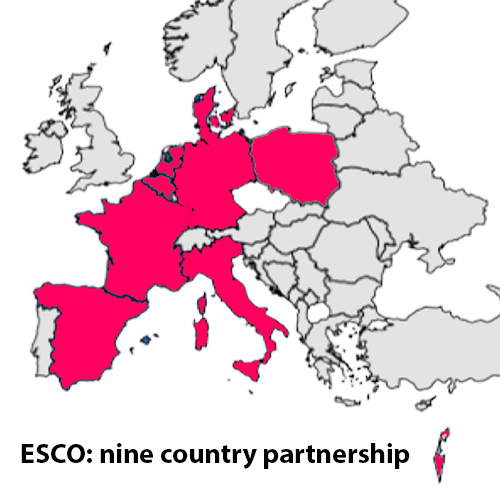
In 2021, Prof. Verhage established the European STXBP1 Consortium (ESCO) together with Ganna Balagura (Gaslini Hospital, Genoa, Italy), marking a significant step towards enhancing clinical trial readiness for potential STXBP1-RD treatments. The consortium includes academic and industry members from eight European countries plus Israel, ESCO’s mission is to ensure efficient evaluation of new therapies and promote equitable, evidence-based access to these innovations.
With its first funding secured from the international STXBP1 Foundation and backing from industry partners, ESCO is set to start a natural history study across EU sites and Israel in early 2024. A natural history study in medical research aims to gather comprehensive data about the progression of a disease over time, in the absence of therapeutic intervention. This type of study helps researchers understand how a disease develops and affects patients, providing valuable baseline data.
A foundation for future clinical trials
ESCO has also applied to the European Medicines Agency (EMA) Innovative Tools program. This step aims to enhance the use of data from the natural history study by integrating it as a concurrent control arm in upcoming clinical trials. By doing so, ESCO intends to streamline the trial process and increase the efficiency and effectiveness of testing new treatments.
Achieving hope and novel solutions
ESCO exemplifies a collaborative effort uniting clinicians, researchers, families, and industry to accelerate the creation of life-changing solutions for patients with STXBP1-RD. By forming a solid foundation for understanding the natural progression of STXBP1-related conditions and leveraging this knowledge in the design and execution of future clinical trials, it underscores the power of collective action in achieving hope and novel solutions for this patient population.
Additionally, Amsterdam UMC has been hosting STXBP1-RD patient family days since 2018, fostering community and support among affected families.
For more details on ESCO’s endeavors, visit www.stxbp1eu.org.